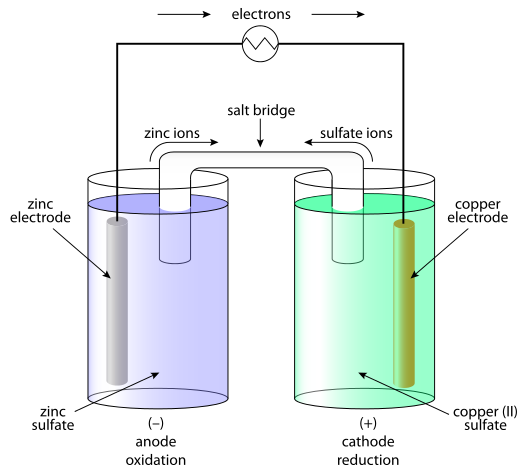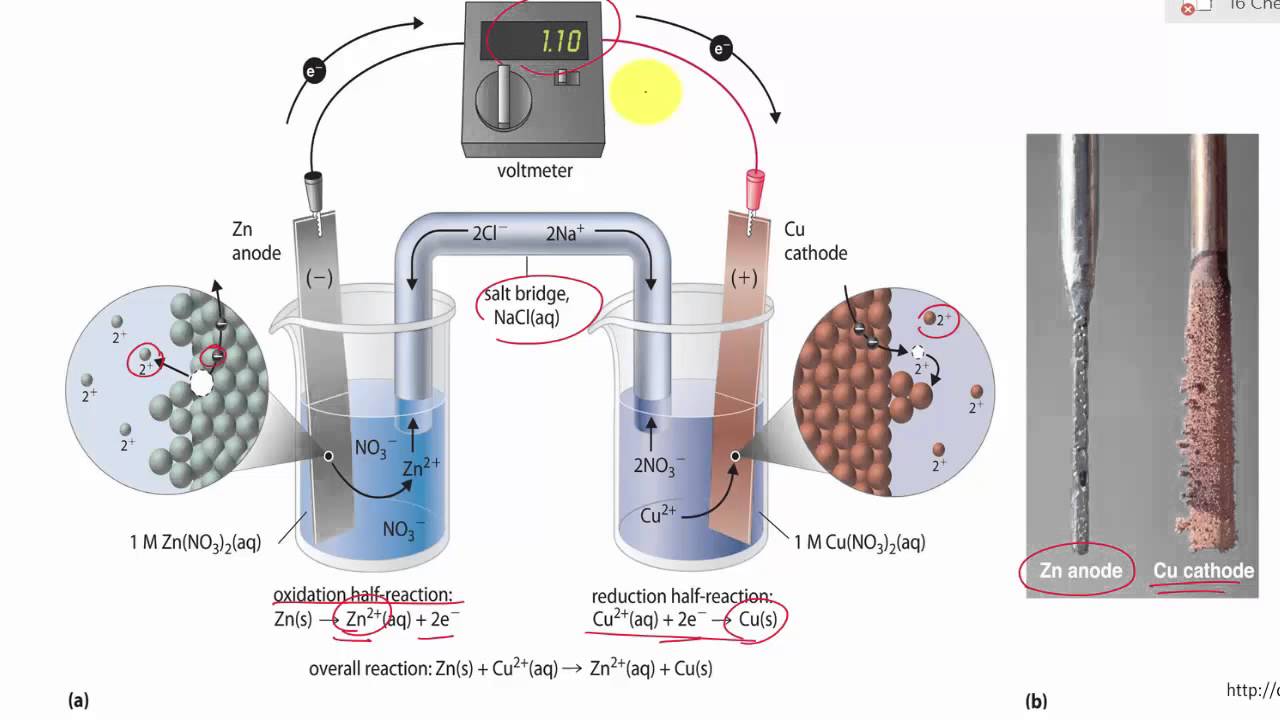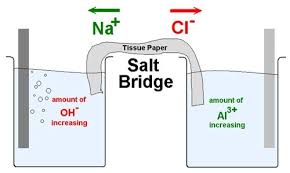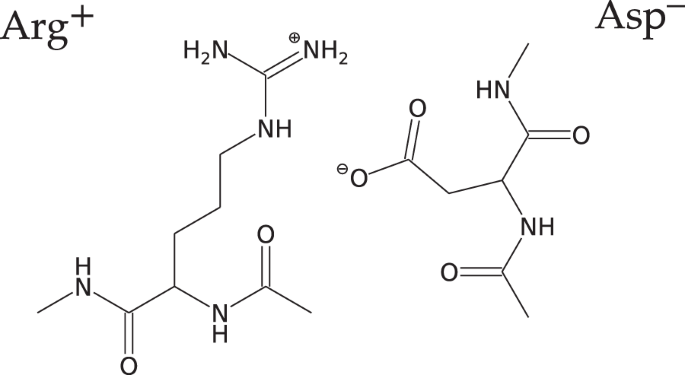
Salt Bridge in Aqueous Solution: Strong Structural Motifs but Weak Enthalpic Effect | Scientific Reports
Salt bridge, 90 mm x 90 mm, 20 mm diam. - Standard potentials of metals using stand material - Electrochemical potentials - Electrochemistry - Physical chemistry - Catalogue of experiments - Chemistry - Chemistry

Free shipping agar salt bridge U-shaped glass tube salt bridge primary battery experiment chemical experiment

Manganese and copper voltaic cell. Copper (right) and manganese (left) half cells joined by a salt bridge. When a stick of copper (Cu) is inserted in Stock Photo - Alamy